China's domestically produced anti-cancer drug with 600 billion in sales achieves astonishing efficacy first in Chinese patients!
In the late autumn of 2024, 56-year-old Uncle Chen sat in the corridor of Sun Yat-sen University Cancer Center, clutching the follow-up report he had just received. The diagnosis of recurrent and metastatic nasopharyngeal cancer weighed on him like a massive boulder, making it hard to breathe—this was his third battle with cancer. The first time, surgery removed the primary tumor; the second time, radiotherapy controlled the localized lesion. Now, the cancer cells had spread through his lymph nodes to his liver.
"The doctor said chemotherapy could only last for three months at most. We tried the immunotherapy combination, but the tumor still grew." Uncle Chen's voice was hoarse, "I even secretly wrote a will, thinking maybe I should just stop treatment......"
Until December 2024, he became one of the first globally to receive iza-bren (BL-B01D1) treatment. Now, six months later, Uncle Chen's tumor markers have dropped by 70%, with imaging showing his liver lesions shrinking by nearly half.
Touching his no-longer-painful shoulder, he smiled: "I never thought a drug developed here in China could pull me back from death's door."

Stories like this unfold daily in the clinical trial wards of Biotheus.
On November 21, 2025, the Center for Drug Evaluation (CDE) of the National Medical Products Administration officially accepted the market application for the bispecific ADC drug iza-bren (BL-B01D1), developed by Baili-Heng, for the treatment of locally advanced or metastatic nasopharyngeal carcinoma.
The data comes from iza-bren—in the latest Phase III clinical study, the drug achieved a 54.6% objective response rate and a median progression-free survival exceeding 8 months in treating recurrent or metastatic nasopharyngeal carcinoma as a later-line therapy.
What does this mean? It means a new lifeline has been forcibly carved out for nasopharyngeal cancer patients who had no available treatments.
After years of waiting, nasopharyngeal cancer patients have finally welcomed a new drug. For Zhu Yi, the helmsman of Baili-Heng who recently became Sichuan's richest person, this marks the beginning of this Chinese-origin drug taking center stage in the global arena of tumor-targeted therapy.
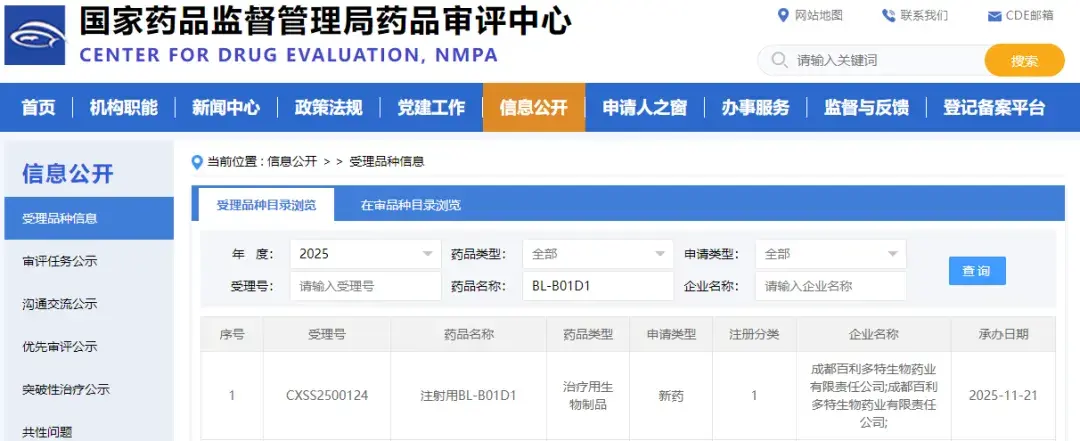
◎ Bailiheng's bispecific antibody-drug conjugate (ADC) is expected to be the first to launch in China by 2026. / Image: National Medical Products Administration
"Master Key": A Drug’s Battle Against Multiple Cancers
Nasopharyngeal carcinoma, a common head and neck malignancy in China, is particularly prevalent in the southern regions. Although radiotherapy is the primary treatment, 20%-30% of patients still experience recurrence after treatment or are diagnosed with distant metastasis at initial diagnosis.
For these patients, immunotherapy combined with chemotherapy is the standard first-line treatment. However, even with this approach, only 20%-30% of patients achieve long-term disease control.
"For the remaining 70% of patients, there are very limited options for second-line treatment," Professor Zhang Li from the Department of Medical Oncology at Sun Yat-sen University Cancer Center candidly stated during an interview. "Subsequent use of chemotherapy drugs not only yields low response rates but also provides very short periods of benefit, with most patients surviving only 1.5 to 5.3 months."
What should be done for these patients?
The emergence of iza-bren has completely broken this deadlock.

◎ The Phase 3 clinical research results on iza-bren for nasopharyngeal carcinoma conducted by Professor Zhang Li were published in the internationally renowned medical journal *The Lancet*. / Image: *The Lancet* official website
The latest Phase III clinical trial by Professor Zhang Li's team confirmed that the median overall survival of such patients extended to 8.38 months after using the drug, while the objective response rate significantly increased from approximately 20% to 54.6%. "Such data performance indeed exceeded our expectations," Professor Zhang Li stated.
Even more astonishingly, iza-bren acts like a "master key," with nasopharyngeal cancer being just the first door it unlocks.
At the European Society for Medical Oncology (ESMO) conference held in October this year, iza-bren unveiled remarkable data in the field of ovarian cancer—demonstrating an objective response rate of 49.0% among the entire population of recurrent ovarian cancer patients (including both platinum-sensitive and platinum-resistant cases), effectively delaying disease recurrence.
Shortly afterward, at the World Conference on Lung Cancer (WCLC), iza-bren delivered another breakthrough: when combined with osimertinib as a first-line treatment for EGFR-mutated non-small cell lung cancer, tumors in all patients showed shrinkage—a 100% objective response rate, hailed as a "miracle" in the history of lung cancer treatment.
39 Deep Breath learned that iza-bren is currently undergoing more than 40 clinical trials in China and the U.S. targeting various tumor types. As of now, the drug has had seven indications included in the CDE's Breakthrough Therapy list, one indication prioritized for review by the CDE, and one indication designated as a Breakthrough Therapy by the U.S. FDA.
Its covered indications include nasopharyngeal carcinoma, non-small cell lung cancer, small cell lung cancer, breast cancer, esophageal squamous cell carcinoma, gastric cancer, and other solid tumors.
ADC Drugs: Why They're Called "Precision Missiles" Against Cancer
Like PD-1/PD-L1 inhibitors before them, ADC drugs have recently become the "hot new thing" in the field of cancer treatment.
Dr. Yang Yunpeng, Chief Physician of Medical Oncology at Sun Yat-sen University Cancer Center, explains that ADC drugs consist of three key components: an antibody, a linker, and a small-molecule toxin. The antibody precisely targets tumor cells; the linker delivers the toxin and releases the small-molecule toxin in the appropriate environment; once inside the cancer cell, the small-molecule toxin directly kills the cell.
"Unlike chemotherapy, which damages healthy cells while killing cancer cells, ADC drugs can deliver cytotoxic agents directly inside tumor cells, significantly reducing harm to healthy tissues," Yang Yunpeng analogizes. "More importantly, when the cytotoxic drug is released, some of it diffuses to surrounding cells that don't express the antigen targeted by the ADC, triggering a 'bystander effect.' That's why it's called a 'magic bullet' in the industry."
In 2000, the first ADC drug was launched in the United States, achieving stunning results in the field of hematologic malignancies and quickly being hailed as a "super miracle drug" in cancer treatment.

◎ The first bispecific antibody-drug conjugate (ADC) to complete global Phase 3 clinical trials worldwide. / Image: Baili Hengtian official website
The success of ADC drugs in Europe and America has acted as a catalyst, sparking explosive growth in China. According to the Wisdom Garden New Drug Intelligence Database, by 2025, China will have conducted over 1,000 ADC drug clinical trials, involving more than a hundred pharmaceutical companies, positioning China as a hub for ADC drug development.
However, traditional ADC drugs face a critical drawback: their single-target design is prone to failure due to tumor heterogeneity. Iza-bren, as the world's first bispecific ADC, simultaneously targets EGFR and HER3, two receptors highly expressed in solid tumors.
"It's like equipping a missile with a dual guidance system—it can cover more tumor cells while reducing off-target toxicity," Yang Yunpeng vividly explained.
This innovative design has set Bailiheng apart in the fiercely competitive ADC field. At the end of 2023, American pharmaceutical giant Bristol-Myers Squibb (BMS) partnered with Bailiheng for a total deal worth $8.4 billion, with an upfront payment of $800 million—a record-breaking sum for a Chinese ADC drug's global expansion.
According to Essence Securities statistics, from 2021 to May 2023, the transaction value of domestically produced ADC drugs going global has exceeded $20 billion. Cross-border pharmaceutical companies frequently "shopping" for ADC pipelines in China has become the industry's new norm.

◎ ADC drugs are hailed as "magic bullets" in the field of tumor-targeted therapy. / Photo: Panoramic Vision
From generic drugs to innovative drugs, Baili-Heng's "big gamble"
It's hard to imagine that Baili-Heng, now holding the world's first bispecific ADC for solid tumors, was a "veteran of generic drugs" a decade ago, struggling with centralized procurement losses and an aging pipeline.
"Li Tianheng invested almost every penny earned from generic drugs into innovative drug R&D," recalled founder Zhu Yi. "With shrinking profits in generics, we had to bet on innovation." While domestic pharmaceutical companies were still entangled in PD-1 and well-established targets, he defied opposition and poured all the profits from generics into high-difficulty bispecific ADC—an area even multinational pharma giants approach cautiously.
The 2022 annual report showed that among Baili Tianheng's 50 R&D projects, 23 were innovative biologics, with 4 ADCs entering clinical trials, led by iza-bren.
This "all-or-nothing" strategy propelled Baili Tianheng's market value to exceed 100 billion by 2025 and elevated Zhu Yi to the Hurun Rich List as the wealthiest individual in Sichuan.
Yet doubts have never disappeared: "Big pharmaceutical companies like Hengrui have opted for a 'light-asset' approach to global expansion, but what gives Baili Tianheng the confidence to bear the full weight of global R&D themselves?" The answer lies in Zhu Yi's ambition: "We're not after quick profits—we aim to become a multinational pharmaceutical company with global pricing power." Unlike the traditional "New-Co" model, Baili Tianheng has chosen to jointly shoulder development costs and share global rights—meaning that if successful, the company will take a sizable slice of the sales pie in the future.
Many people are asking whether Bili Tianheng will become the "next Hengrui."
Hengrui Medicine is an unavoidable giant in China's innovative drug industry. The two companies indeed share similar origins, both starting with generic drugs and accumulating initial capital through the "generic-to-innovative" model.
Today, innovative drugs account for over 60% of Hengrui's revenue. In contrast, the challenges facing Bili Tianheng are ever-present.
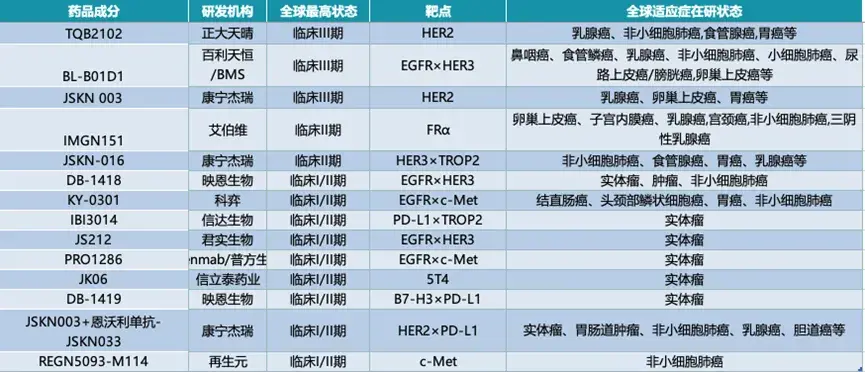
◎ Several domestic pharmaceutical companies in China are conducting clinical research on ADCs. / Image: Screenshot from the internet
After iza-bren hits the market in 2026, commercialization will be the first major hurdle to overcome.
The global ADC market has already surpassed $16 billion, with 20 drugs competing simultaneously. Baili Tianheng not only faces direct competition from international giants like Roche and AstraZeneca but also contends with domestic rivals such as Kangfang and Innovent.
"Blockbuster products can support market value, but multinational pharmaceutical companies need a sustainable product pipeline," cautioned pharmaceutical expert Du Chen. Currently, among Baili-Hengtian's ADC pipeline, candidates targeting gastric cancer and esophageal squamous cell carcinoma have entered Phase II trials. "If the follow-up pipeline fails to keep up, the company risks facing the dilemma of 'first-drug dependency.'"
But for patients like Uncle Chen, a more realistic expectation is that iza-bren can be included in medical insurance as soon as possible, making it affordable for more cancer patients who have "no treatment options." After all, "survival" matters more than "being domestically developed in China."
In any case, the first-to-market launch of Iza-Bren isn't just the victory of a single drug—it represents a major milestone in China's pharmaceutical innovation, proving that we're no longer merely a "generic drug powerhouse" but also capable of developing original drugs that redefine global treatment protocols.
More importantly, it has brought new hope to countless Chinese cancer patients, offering them a glimpse of breaking free from the dilemma of "unaffordable and inaccessible" treatments.
Of course, we must soberly recognize that iza-bren is not a "miracle cure," and whether the pipeline can continue to deliver remains the sword of Damocles hanging over the company's head.
The test of time has only just begun. But at least now, an increasing number of "Uncle Chens" can see a very real light during their darkest hours.
And this light comes from China.